Invited Speakers:Prof. Olivier Couture, Mr. Vincent Hingot, Mr. Baptiste Heiles
Institut Langevin, CNRS, INSERM, ESPCI Paris, PSL Research University, 17 rue Moreau, 75012, Paris.
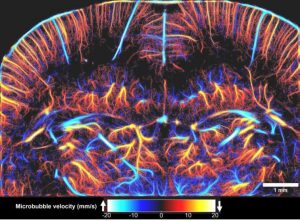
We have shown that such phenomenon can also be obtained in ultrasound imaging by observing large concentrations of microbubbles at ultrafast frame rates. This lead us to introduce the concept of ultrafast ultrasound localization microscopy [uULM, Couture et al. Patent 2010, Couture et al. IEEE IUS Orlando 2011, Couture et al. IEEE UFFC 2018], which allows super-resolution imaging by reconstructing the precise position of individual microbubbles flowing into microvessels. We initially tested ULM in 3D in a microvessel phantom [Desailly et al. APL, 2013], before moving to the rat brain[Errico et al. Nature, 2015]. This technique has recently been applied by Dayton’s group to map angiogenesis in a tumor model and assess its tortuosity [Lin et al. 2016].
Other approaches for ultrasound super-resolution have since been proposed. Microbubble Accumulation Super Resolution, which relies on low dilution of microbubbles observed at conventional frame rates has been applied both in-vitro [O’Reilly et al, Medical Physics 2013; Viessmann et al., PMB, 2013] and in-vivo [Siepmann et al., IEEE IUS 2011, Christensen-Jeffries et al., IEEE UFFC, 2015]. More recently, Super-Resolution Acoustical Fluctuation Imaging, which relies on the higher-order statistics of the echoes temporal fluctuation, has been demonstrated by Bar-Zion et al. [ArXiv 2016]. In general, the position of individual microbubbles can be determined with a resolution defined by the signal to noise ratio and the geometry of the system [Desailly et al., PMB, 2015]. The maximum resolution attainable with current programmable scanners was predicted to be 5 microns at 15 MHz, or wavelength/20. In uULM, this was confirmed in-vivo by injecting boluses of microbubbles in rat brain and performing ultrafast imaging coronally (500 fps) for 150 seconds. The resulting map of microbubble positions was reconstructed with a resolution of λ/12 (8 μm), down to 12 mm in depth. Ultrasound localization microscopy brings new parameters in the trade-off between penetration and resolution, such as the concentration of microbubbles, frame rate, signal-to-noise ratio and acquisition time. This approach allows us to map vessels that are not visible by other imaging modalities in-depth. However, it remains limited by micrometric motion which can drastically affect image quality. Subwavelength image registration has been proposed to solve this issue [Hingot et al., Ultrasonics, 2016a]. Data overload, challenging probe design and electronics, currently restrict the use of 3D uULM, which is a conditio sine qua non for the mapping of complex microvasculature. Finally, we consider that ultrasound therapy should also benefit from the evolution toward a subwavelength precision, which was recently demonstrated for drug-delivery [Hingot et al., APL, 2016b].
Bio-sketch: Olivier Couture

Olivier Couture was born in Quebec City (Canada) in 1978. He received his B.Sc degree in physics from McGill University, Montreal, Canada, in 2001, and his Ph.D degree from the department of Medical Biophysics, University of Toronto, Canada, in 2007. After a postdoctoral fellowship at ESPCI in Paris (France), he was hired as a tenured research associate at CNRS, based within the Langevin Institute. He was awarded the “2017 IEEE Ultrasonics Early Career Investigation Award” for the development of ultrasound super-resolution and plane-wave contrast imaging and the Sylvia Sorkin Greenfield Award of the American Association of Physicists in Medicine for the Best Paper published in Medical Physics in 2011. He received the prestigious ERC Consolidator Grant for the application of ultrasound localization microscopy to medical diagnosis. His current research interests include ultrasound localization microscopy, ultrafast ultrasound imaging, drug delivery, velocipede oscillations, contrast agents, diabetes and stroke.
Title: Ultrasound Localization Microscopy In Vivo
Abstract:By localizing the acoustic signature of individual microbubbles in the vasculature, Ultrasound Localization Microscopy (ULM) surpasses the diffraction-limit by orders of magnitude and reaches microscopic resolution. In Errico et al, Nature 2015, ULM achieved resolution of 8 μm over a depth of 12 mm in the rat brain. However, to reach such a microscopic resolution, more than one million microbubbles had to be individually localized and tracked for three minutes, highlighting the fundamental trade-off between temporal and spatial resolutions. Significant progress has been made in image processing strategies to correct motion induced artifacts, to increase localization precision and to reduce computation time. However, because of its intrinsic link with the vasculature, ULM still faces an incompressible compromise between the injected microbubble dose, the acquisition time and the spatial resolution. ULM was performed to image the cerebral microvasculature in rats. Six million microbubbles were localized with a precision of 5 μm. Saturation curves were obtained for images with pixel sizes ranging from 5 μm to 100 μm. Additionally, a detailed time analysis of the reconstruction was performed in 150 individualized vessels. We show that, while 90μm vessels are reconstructed in seconds, it takes several minutes to map the vessels at the scale of the capillaries (5 μm). The fundamental limit of spatial resolution is determined through a model linking the necessary acquisition time with the microbubble’s concentration, the pixel size and the size of the vessels to reconstruct. This model should help to understand both capabilities and limitations of ULM for the description of the microvasculature.
Bio-sketch: Vincent Hingot
Vincent Hingot graduated from ESPCI in 2016, a French engineering school located in Paris, majoring in engineering technologies for health. He started a PhD in Institute Langevin shortly after to work on medical ultrasounds with Mickael Tanter and Olivier Couture. Following the work of Claudia Errico and Marine Bezagu, he worked on exploiting different interactions between ultrasound and contrast agents for imaging and therapy. He focused mainly on the development of ultrafast Ultrasound Localization Microscopy (ULM) and its application to in vivo study of different vascular pathologies, together with the development of new techniques for ultrasound mediated drug delivery and its application to the delivery of anti-cancerous agents.
Title: Volumetric ultrafast Ultrasound Localisation Microscopy
Abstract:
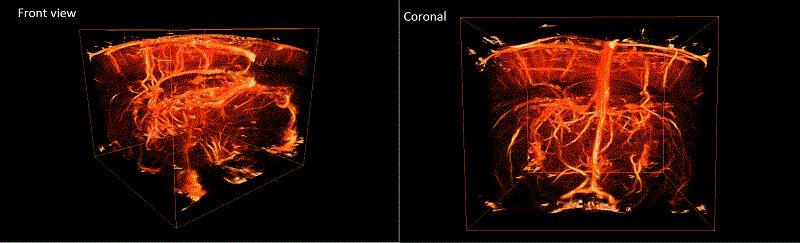
Ultrasound localization microscopy has first been demonstrated to overcome the penetration/resolution paradigm [Couture et al IEEE IUS 2011]. Inspired by FPALM in optics and exploiting ultrafast ultrasound imaging, it allowed the reconstruction of a full velocity map of the rat brain vasculature with a micrometric resolution (8 μm) [Errico et al. Nature,2015]. Despite additional successes for tumour imaging [Lin et al, Theranostics, 2017], this plane-by-plane technique suffers from minute-long acquisitions, out-of-plane microbubble and tissue motion which cannot be corrected for [Hingot et al. Ultrasonics 2017] and the loss of information due to the projection of a 3D vascular structure into a 2D image. We’ve successfully demonstrated the possibility to use an isotropic matrix array for 3D in vitro superresolution imaging at high frame rates. We present here the first application of this process in vivo. The 2D matrix array with 1024 elements arranged in a 32x32 isotropic plane was controlled by the customised programmable 1024-channel system presented in [Provost et al. 2014]. Sprague Dawley rats underwent craniotomy surgery. Sonovue microbubbles were injected through a catether in boli of 0.2mL while a Ketamine-Domitor solution was perfused. The brain was insonified with 9MHz plane waves inclined at 12 different angles with a compounded frame rate of up to 500 Hz. The 3D ULM process developed in [Heiles et al, submitted] was used to determine the position of the bubbles, track them and measure velocities. After implementation of this technique on 99900 volumes, a volumetric rendering of rat brain microvasculature was obtained. At 9MHz, the conventional resolution with this 2D array is around 250μm. The theoretical resolution obtainable with the technique proposed here is less than 1μm in the axial direction and around 5μm in lateral and elevational directionTrajectories calculated allowed to yield velocity fields in microvessels of a few cm/s. Volumetric ULM allows to surpass the conventional resolution in 3 dimensions in a large volume with only 200s of acquisitions, however, the limited sensitivity of 2D matrix arrays, the data size, computation and transfer times remain major challenges.
Bio-sketch: Baptiste Heiles
Baptiste Heiles was born in France in 1990. He received a Master of Science in General and Civil Engineering from Ecole Normale Superieure Paris Saclay in 2013, the agrégation de Sciences Industrielles in 2014, and a Master of Research in Fluid Mechanics and Heat Transfers from Ecole Centrale de Paris in 2015. Following the work of Yann Desailly and Claudia Errico, he works on exploiting contrast agents for imaging. He focuses on implementing Ultrasound Localization Microscopy (uULM) in volumetric imaging as well as improving ULM in conventional 2D imaging.